Complex Regulation of the Tir1/afb Family of Auxin Receptors

Indole-3-acetic acid (IAA) is the about arable and the basic auxin natively occurring and functioning in plants. It generates the majority of auxin furnishings in intact plants, and is the most strong native auxin.
At that place are four more endogenously synthesized auxins in plants.[1] [ii]
All auxins are compounds with aromatic ring and a carboxylic acid group:[2] [3]

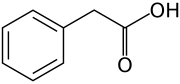


Auxins (plural of auxin ) are a form of plant hormones (or plant-growth regulators) with some morphogen-like characteristics. Auxins play a cardinal function in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their function in plant growth in the 1920s.[four] Kenneth V. Thimann became the first to isolate one of these phytohormones and to decide its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.
Overview [edit]
Auxins were the first of the major found hormones to be discovered. They derive their proper name from the Greek discussion αυξειν (auxein – "to grow/increase"). Auxin is present in all parts of a plant, although in very different concentrations. The concentration in each position is crucial developmental information, so it is subject to tight regulation through both metabolism and transport. The result is the auxin creates "patterns" of auxin concentration maxima and minima in the plant body, which in plow guide farther development of respective cells, and ultimately of the plant as a whole.
The (dynamic and environment responsive) design of auxin distribution within the plant is a key factor for plant growth, its reaction to its surround, and specifically for development of establish organs[5] [6] (such as leaves or flowers). It is achieved through very complex and well-coordinated active transport of auxin molecules from jail cell to prison cell throughout the constitute trunk — by the so-called polar auxin transport.[five] Thus, a constitute tin (equally a whole) react to external atmospheric condition and suit to them, without requiring a nervous system. Auxins typically deed in concert with, or in opposition to, other plant hormones. For instance, the ratio of auxin to cytokinin in certain institute tissues determines initiation of root versus shoot buds.
On the molecular level, all auxins are compounds with an aromatic ring and a carboxylic acrid grouping.[iii] The most important fellow member of the auxin family unit is indole-3-acetic acid (IAA),[7] which generates the bulk of auxin effects in intact plants, and is the most potent native auxin. And as native auxin, its equilibrium is controlled in many ways in plants, from synthesis, through possible conjugation to deposition of its molecules, always according to the requirements of the state of affairs.
- V naturally occurring (endogenous) auxins in plants include indole-3-acetic acid, 4-chloroindole-3-acerb acrid, phenylacetic acrid, indole-3-butyric acid, and indole-3-propionic acid.[1] [2] Yet, most of the knowledge described then far in auxin biology and as described in the sections which follow, apply basically to IAA; the other 3 endogenous auxins seems to have marginal importance for intact plants in natural environments. Alongside endogenous auxins, scientists and manufacturers take developed many constructed compounds with auxinic activeness.
- Constructed auxin analogs include 1-naphthaleneacetic acid, 2,4-dichlorophenoxyacetic acid (2,4-D),[1] and many others.
Some synthetic auxins, such equally ii,4-D and 2,four,5-trichlorophenoxyacetic acid (two,4,5-T), are sold as herbicides. Broad-leaf plants (dicots), such as dandelions, are much more susceptible to auxins than narrow-leaf plants (monocots) such as grasses and cereal crops, making these constructed auxins valuable equally herbicides.
Discovery of auxin [edit]
Charles Darwin [edit]
In 1881, Charles Darwin and his son Francis performed experiments on coleoptiles, the sheaths enclosing young leaves in germinating grass seedlings. The experiment exposed the coleoptile to light from a unidirectional source, and observed that they bend towards the light.[viii] By covering various parts of the coleoptiles with a low-cal-impermeable opaque cap, the Darwins discovered that light is detected past the coleoptile tip, just that bending occurs in the hypocotyl. Notwithstanding the seedlings showed no signs of evolution towards light if the tip was covered with an opaque cap, or if the tip was removed. The Darwins ended that the tip of the coleoptile was responsible for sensing light, and proposed that a messenger is transmitted in a downwards direction from the tip of the coleoptile, causing it to bend.[nine]
Peter Boysen Jensen [edit]
In 1910, Danish scientist Peter Boysen Jensen demonstrated that the phototropic stimulus in the oat coleoptile could propagate through an incision.[10] These experiments were extended and published in greater item in 1911 and 1913.[11] [12] He found that the tip could be cut off and put back on, and that a subsequent one-sided illumination was nonetheless able to produce a positive phototropic curvature in the basal part of the coleoptile. He demonstrated that the transmission could take place through a thin layer of gelatin separating the unilaterally illuminated tip from the shaded stump. By inserting a piece of mica he could block manual in the illuminated and non-illuminated side of the tip, respectively, which immune him to prove that the transmission took place in the shaded part of the tip. Thus, the longitudinal half of the coleoptile that exhibits the greater rate of elongation during the phototropic curvature, was the tissue to receive the growth stimulus.[xiii] [14]
In 1911, Boysen Jensen ended from his experimental results that the transmission of the phototropic stimulus was not a physical effect (for example due to a modify in force per unit area) simply serait dû à une migration de substance ou d'ions (was caused by the ship of a substance or of ions).[xi] These results were fundamental for further piece of work on the auxin theory of tropisms.
Frits Went [edit]
In 1928, the Dutch botanist Frits Warmolt Went showed that a chemical messenger diffuses from coleoptile tips. Went's experiment identified how a growth promoting chemical causes a coleoptile to grow towards the light. Went cut the tips of the coleoptiles and placed them in the night, putting a few tips on agar blocks that he predicted would absorb the growth-promoting chemical. On command coleoptiles, he placed a block that lacked the chemic. On others, he placed blocks containing the chemic, either centered on elevation of the coleoptile to distribute the chemical evenly or outset to increase the concentration on ane side.[9]
When the growth-promoting chemical was distributed evenly the coleoptile grew directly. If the chemic was distributed unevenly, the coleoptile curved away from the side with the cube, as if growing towards the light, even though it was grown in the nighttime. Went later proposed that the messenger substance is a growth-promoting hormone, which he named auxin, that becomes asymmetrically distributed in the angle region. Went ended that auxin is at a higher concentration on the shaded side, promoting prison cell elongation, which results in coleoptiles angle towards the light.[14]
Hormonal activity [edit]
Auxins assist development at all levels in plants, from the cellular level, through organs, and ultimately to the whole plant.
Molecular mechanisms [edit]
When a constitute cell comes into contact with auxin, it causes dramatic changes in factor expression, with many genes up- or downwards-regulated. The precise mechanisms past which this occurs are withal an area of active research, but in that location is now a full general consensus on at least two auxin signalling pathways.[15] [16]
Perception [edit]
The most well-characterized auxin receptors are the TIR1/ AFB family of F-box proteins. F-box proteins target other proteins for deposition via the ubiquitin deposition pathway. When TIR1/ AFB proteins bind to auxin, the auxin acts as a 'molecular glue' that allows these proteins to then bind to their targets (see below).
Some other auxin-binding protein, ABP1 is now often regarded as an auxin receptor (at the apoplast), but it is generally considered to have a much more minor office than the TIR1/AFB signaling pathway, and much less is known about ABP1 signaling.[16]
Aux/IAA and ARF signalling modules [edit]

The auxin bespeak pour: In the absenteeism of auxin, Aux/IAA bind to and suppress the transcriptional activity of ARFs. When auxin is present it forms a 'molecular glue' between TIR1 and Aux/IAAs, which leads to the degradation of these repressors. ARFs are and then free to bind to DNA and to cause changes in transcription.
Auxin response factors (ARFs) are a large group of transcription factors that act in auxin signaling. In the absence of auxin, ARFs bind to a class of repressors known as Aux/IAAs. Aux/IAA suppress the ability of ARFs to enhance gene transcription. Additionally, the bounden of Aux/IAA to ARFs brings Aux/IAA into contact with the promoters of auxin-regulated genes. When at these promoters, Aux/IAA repress the expression of these genes through recruiting other factors to make modifications to the Dna structure.
The binding of auxin to TIR1/AFBs allows them to bind to Aux/IAAs. When bound by TIR1/AFBs, Aux/IAAs are marked for degradation. The deposition of Aux/IAA frees ARF proteins, which are then able to activate or repress genes at whose promoters they are spring.[xv] [16]
The large number of Aux/IAA and ARF bounden pairs possible, and their different distributions between prison cell types and across developmental age are thought to account for the astonishingly diverse responses that auxin produces.
In June 2018, it was demonstrated that plant tissues tin answer to auxin in a TIR1-dependent manner extremely quickly (probably also speedily to be explained by changes in gene expression). This has led some scientists to propose that there is an as yet unidentified TIR1-dependent auxin-signalling pathway that differs from the well-known transcriptional response.[17]
On a cellular level [edit]
On the cellular level, auxin is essential for cell growth, affecting both cell division and cellular expansion. Auxin concentration level, together with other local factors, contributes to jail cell differentiation and specification of the cell fate.
Depending on the specific tissue, auxin may promote axial elongation (as in shoots), lateral expansion (as in root swelling), or iso-diametric expansion (as in fruit growth). In some cases (coleoptile growth), auxin-promoted cellular expansion occurs in the absence of prison cell division. In other cases, auxin-promoted cell division and cell expansion may be closely sequenced inside the same tissue (root initiation, fruit growth). In a living found, auxins and other constitute hormones nearly ever appear to interact to determine patterns of institute development.
Organ patterns [edit]
Growth and division of plant cells together result in the growth of tissue, and specific tissue growth contributes to the development of constitute organs.
Growth of cells contributes to the plant's size, unevenly localized growth produces angle, turning and directionalization of organs- for example, stems turning toward low-cal sources (phototropism), roots growing in response to gravity (gravitropism), and other tropisms originated considering cells on one side grow faster than the cells on the other side of the organ. So, precise control of auxin distribution betwixt unlike cells has paramount importance to the resulting form of constitute growth and organization.
Auxin send and the uneven distribution of auxin [edit]
To cause growth in the required domains, auxins must of necessity be active preferentially in them. Local auxin maxima can exist formed by active biosynthesis in sure cells of tissues, for example via tryptophan-dependent pathways,[18] merely auxins are non synthesized in all cells (even if cells retain the potential ability to exercise then, just under specific conditions will auxin synthesis be activated in them). For that purpose, auxins accept to exist non only translocated toward those sites where they are needed but as well they must have an established machinery to detect those sites. Translocation is driven throughout the plant torso, primarily from peaks of shoots to peaks of roots (from upwardly to downwards).
For long distances, relocation occurs via the stream of fluid in phloem vessels, but, for short-distance transport, a unique organisation of coordinated polar transport directly from jail cell to cell is exploited. This curt-distance, agile transport exhibits some morphogenetic properties.
This process, polar auxin transport, is directional, very strictly regulated, and based in uneven distribution of auxin efflux carriers on the plasma membrane, which ship auxins in the proper direction. While PIN-FORMED (PIN) proteins are vital in transporting auxin in a polar mode,[six] [xix] the family of AUXIN1/Like-AUX1 (AUX/LAX) genes encodes for non-polar auxin influx carriers.[20]
The regulation of PIN protein localisation in a cell determines the management of auxin send from cell, and full-bodied endeavor of many cells creates peaks of auxin, or auxin maxima (regions having cells with higher auxin – a maximum).[6] Proper and timely auxin maxima within developing roots and shoots are necessary to organise the development of the organ.[5] [21] [22] PINs are regulated by multiple pathways, at both the transcriptional and the mail service-translational levels. PIN proteins can be phosphorylated past PINOID, which determines their apicobasal polarity and thereby the directionality of auxin fluxes. In addition, other AGC kinases, such as D6PK, phosphorylate and actuate Pivot transporters. AGC kinases, including PINOID and D6PK, target to the plasma membrane via binding to phospholipids. Upstream of D6PK, iii'-phosphoinositide dependent protein kinase 1 (PDK1) acts as a main regulator. PDK1 phosphorylates and activates D6PK at the basal side of plasma membrane, executing the activeness of Pivot-mediated polar auxin transport and subsequent constitute development.[23] Surrounding auxin maxima are cells with depression auxin troughs, or auxin minima. For example, in the Arabidopsis fruit, auxin minima have been shown to be important for its tissue development.[24]
Auxin has a significant effect on spatial and temporal factor expressions during the growth of apical meristems. These interactions depend both on the concentration of Auxin as well as the spatial orientation during primordial positioning. Auxin relies on PIN1 which works equally an auxin efflux carrier. PIN1 positioning upon membranes determines the directional menstruum of the hormone from college to lower concentrations.[25] Initiation of primordia in apical meristems is correlated to heightened auxin levels.[26] Genes required to specify the identity of cells arrange and limited based on levels of auxin. STM (SHOOTMERISTEMLESS), which helps maintain undifferentiated cells, is down-regulated in the presence of auxin. This allows growing cells to differentiate into various plant tissues. The CUC (CUP-SHAPED COTYLEDON) genes set the boundaries for growing tissues and promote growth.[27] They are upregulated via auxin influx.[28] Experiments making use of GFP (Dark-green FLUORESCENCE PROTEIN) visualization in Arabidopsis have supported these claims.
Arrangement of the establish [edit]
As auxins contribute to organ shaping,[five] [6] they are also fundamentally required for proper development of the plant itself.[5] Without hormonal regulation and organization, plants would exist but proliferating heaps of similar cells. Auxin employment begins in the embryo of the plant, where the directional distribution of auxin ushers in subsequent growth and development of primary growth poles, then forms buds of future organs. Side by side, it helps to coordinate proper development of the arising organs, such every bit roots, cotyledons, and leaves and mediates long-distance signals betwixt them, contributing so to the overall compages of the plant.[5] Throughout the plant's life, auxin helps the found maintain the polarity of growth,[5] and actually "recognize" where it has its branches (or whatsoever organ) connected.
An important principle of plant organisation based upon auxin distribution is apical dominance, which ways the auxin produced past the apical bud (or growing tip) diffuses (and is transported) downwards and inhibits the development of ulterior lateral bud growth, which would otherwise compete with the upmost tip for calorie-free and nutrients. Removing the upmost tip and its suppressively acting auxin allows the lower dormant lateral buds to develop, and the buds between the leafage stem and stem produce new shoots which compete to become the lead growth. The process is actually quite complex because auxin transported down from the lead shoot tip has to collaborate with several other found hormones (such equally strigolactones or cytokinins) in the procedure on various positions along the growth axis in plant body to achieve this phenomenon. This institute behavior is used in pruning by horticulturists.
Finally, the sum of auxin arriving from stems to roots influences the degree of root growth. If shoot tips are removed, the plant does non react only past the outgrowth of lateral buds — which are supposed to replace to original lead. Information technology besides follows that smaller corporeality of auxin arriving at the roots results in slower growth of roots and the nutrients are after in higher caste invested in the upper part of the plant, which hence starts to grow faster.
Effects [edit]

A healthy Arabidopsis thaliana plant (left) next to an auxin signal-transduction mutant with a repressed response to auxin.

Auxin participates in phototropism, geotropism, hydrotropism and other developmental changes. The uneven distribution of auxin, due to environmental cues, such as unidirectional light or gravity forcefulness, results in uneven found tissue growth, and more often than not, auxin governs the form and shape of the plant body, direction and strength of growth of all organs, and their mutual interaction.[6] When the cells grow larger, their volume increases every bit the intracellular solute concentration increases with h2o moving into the cells from extracellular fluid. This auxin-stimulated intake of water causes turgor pressure level on the prison cell walls, causing the found to curve.
Auxin stimulates cell elongation by stimulating wall-loosening factors, such equally expansins, to loosen cell walls. The effect is stronger if gibberellins are also present. Auxin too stimulates cell partition if cytokinins are present. When auxin and cytokinin are practical to callus, rooting can be generated with higher auxin to cytokinin ratios, shoot growth is induced by lower auxin to cytokinin ratios, and a callus is formed with intermediate ratios, with the exact threshold ratios depending on the species and the original tissue. Auxin besides induces saccharide and mineral aggregating at the site of awarding.
Wound response [edit]
Auxin induces the formation and system of phloem and xylem. When the plant is wounded, the auxin may induce the cell differentiation and regeneration of the vascular tissues.[29]
Root growth and evolution [edit]
Auxins promote root initiation.[30] Auxin induces both growth of pre-existing roots and root branching (lateral root initiation), and also adventitious root formation. Equally more than native auxin is transported downwardly the stem to the roots, the overall development of the roots is stimulated. If the source of auxin is removed, such as by trimming the tips of stems, the roots are less stimulated accordingly, and growth of stem is supported instead.
In horticulture, auxins, specially NAA and IBA, are commonly practical to stimulate root initiation when rooting cuttings of plants. However, high concentrations of auxin inhibit root elongation and instead enhance accidental root formation. Removal of the root tip tin can lead to inhibition of secondary root formation.
Apical authorisation [edit]
Auxin induces shoot apical authorisation; the axillary buds are inhibited by auxin, as a high concentration of auxin directly stimulates ethylene synthesis in axillary buds, causing inhibition of their growth and potentiation of apical authorization. When the apex of the establish is removed, the inhibitory effect is removed and the growth of lateral buds is enhanced. This is chosen decapitation, usually performed in tea plantations and hedge-making. Auxin is sent to the role of the establish facing away from the lite, where information technology promotes prison cell elongation, thus causing the institute to bend towards the low-cal.[31]
Fruit growth and development [edit]
Auxin is required for fruit growth and development and delays fruit senescence. When seeds are removed from strawberries, fruit growth is stopped; exogenous auxin stimulates the growth in fruits with seeds removed. For fruit with unfertilized seeds, exogenous auxin results in parthenocarpy ("virgin-fruit" growth).
Fruits form aberrant morphologies when auxin ship is disturbed.[32] In Arabidopsis fruits, auxin controls the release of seeds from the fruit (pod). The valve margins are a specialised tissue in pods that regulates when pod will open (dehiscence). Auxin must be removed from the valve margin cells to allow the valve margins to form. This process requires modification of the auxin transporters (PIN proteins).[24]
The evolutionary transition from diploid to triploid endosperms - and the production of converse cells - may have occurred due to a shift in gametophyte evolution which produced a new interaction with an auxin-dependent mechanism originating in the earliest angiosperms.[33]
Flowering [edit]
Auxin plays besides a pocket-sized part in the initiation of flowering and evolution of reproductive organs. In depression concentrations, it can filibuster the senescence of flowers. A number of establish mutants have been described that affect flowering and take deficiencies in either auxin synthesis or transport. In maize, one example is bif2 arid inflorescence2.[34]
Ethylene biosynthesis [edit]
In low concentrations, auxin can inhibit ethylene formation and transport of precursor in plants; notwithstanding, high concentrations can induce the synthesis of ethylene.[35] Therefore, the high concentration can induce femaleness of flowers in some species.[ citation needed ]
Auxin inhibits abscission prior to the formation of the abscission layer, and thus inhibits senescence of leaves.
Synthetic auxins [edit]
In the course of research on auxin biology, many compounds with noticeable auxin action were synthesized. Many of them had been plant to take economical potential for human-controlled growth and evolution of plants in agronomy.
Auxins are toxic to plants in large concentrations; they are well-nigh toxic to dicots and less and then to monocots.[36] Because of this property, synthetic auxin herbicides, including 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,five-trichlorophenoxyacetic acid (2,4,5-T), have been developed and used for weed command.
However, some exogenously synthesized auxins, particularly i-naphthaleneacetic acid (NAA) and indole-3-butyric acid (IBA), are too usually applied to stimulate root growth when taking cuttings of plants or for different agronomical purposes such every bit the prevention of fruit drop in orchards.
Used in high doses, auxin stimulates the production of ethylene, also a native establish hormone. Excess ethylene tin inhibit elongation growth, cause leaves to fall (abscission), and even impale the plant. Some constructed auxins, such as ii,4-D and 2,4,5-T are marketed also equally herbicides. Dicots, such as dandelions, are much more than susceptible to auxins than monocots, such equally grasses and cereal crops. So these synthetic auxins are valuable as synthetic herbicides. two,four-D was the commencement widely used herbicide, and it is nonetheless in use.[37] ii,four-D was first commercialized by the Sherwin-Williams company and saw use in the late 1940s. It is easy and inexpensive to manufacture.
Triclopyr (3,5,six-TPA), while known equally an herbicide, has too been shown to increase the size of fruit in plants. At increased concentrations, the hormone can be lethal. Dosing down to the right concentration has been shown to alter photosynthetic pathways. This hindrance to the plant causes a response that increases carbohydrate production, leading to larger fruit.[38]
Herbicide manufacture [edit]
Synthetic auxins are used as a kind of herbicide and overdosing of auxins will interrupt plants' growth and pb to their death.[39] The defoliant Agent Orange, used extensively past British forces in the Malayan Emergency and American forces in the Vietnam War, was a mix of 2,4-D and ii,four,5-T. The chemical compound two,iv-D is still in use and is thought to be safe, only 2,4,v-T was more or less banned by the U.S. Ecology Protection Agency in 1979. The dioxin TCDD is an unavoidable contaminant produced in the manufacture of ii,4,five-T. As a outcome of the integral dioxin contamination, the use of 2,4,5-T products has been implicated in leukemia, miscarriages, nativity defects, liver impairment, and other diseases.
See also [edit]
- Auxin binding protein
- Fusicoccin
- Herbicide; specifically, run into the section: §Auxin
- Phenoxy herbicide
- Pruning fruit copse
- Tropism
- Witch'southward broom
- Toshio Murashige
- Folke Grand. Skoog
- Kenneth Five. Thimann
References [edit]
- ^ a b c Simon S, Petrášek J (March 2011). "Why plants need more than one type of auxin". Plant Scientific discipline. 180 (3): 454–460. doi:10.1016/j.plantsci.2010.12.007. PMID 21421392.
- ^ a b c Ludwig-Müller J (March 2011). "Auxin conjugates: their role for constitute development and in the evolution of land plants". Periodical of Experimental Botany. 62 (6): 1757–1773. doi:10.1093/jxb/erq412. PMID 21307383.
Besides IAA there are several other molecules with auxin activeness such as indole-iii-butyric acrid (IBA) (Fig. 1), iv-Cl-IAA, and indole-three-propionic acid (IPA). ... The indole moiety (e.thousand. IAA, IBA, IPA, 4-Cl-IAA) as well as the conjugate partner can vary, then that the plant tin can produce many different combinations of conjugates (Bajguz and Piotrowska, 2009). Also, other auxin-blazon molecules such as phenylacetic acid (PAA; Ludwig-Müller and Cohen, 2002) tin can be conjugated (Jentschel et al., 2007)
- ^ a b Taiz L, Zeiger East (1998). Plant Physiology (2d ed.). Massachusetts: Sinauer Assembly.
- ^ Frits Warmolt Went
- ^ a b c d e f thousand Friml J (February 2003). "Auxin transport - shaping the constitute". Current Opinion in Plant Biology. 6 (ane): vii–12. doi:ten.1016/S1369526602000031. PMID 12495745.
- ^ a b c d east Benková E, Michniewicz M, Sauer Grand, Teichmann T, Seifertová D, Jürgens One thousand, Friml J (Nov 2003). "Local, efflux-dependent auxin gradients as a mutual module for found organ germination". Jail cell. 115 (5): 591–602. doi:10.1016/S0092-8674(03)00924-3. PMID 14651850. S2CID 16557565.
- ^ Simon L, Bousquet J, Lévesque RC, Lalonde K (1993). "Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants". Nature. 363 (6424): 67–69. Bibcode:1993Natur.363...67S. doi:10.1038/363067a0. S2CID 4319766.
- ^ Hohm T, Preuten T, Fankhauser C (January 2013). "Phototropism: translating lite into directional growth". American Journal of Botany. 100 (i): 47–59. doi:x.3732/ajb.1200299. PMID 23152332.
- ^ a b Whippo CW, Hangarter RP (May 2006). "Phototropism: bending towards enlightenment". The Found Cell. 18 (5): 1110–1119. doi:x.1105/tpc.105.039669. PMC1456868. PMID 16670442.
- ^ Boysen JP (1910). "Über die Leitung des phototropischen Reizes in Avena-keimpflanzen". Berichte der Deutschen Botanischen Gesellschaft. 28: 118–120. doi:10.1111/j.1438-8677.1910.tb06871.x.
- ^ a b Boysen JP (1911). "La transmission de l'irritation phototropique dans l'Avena" (PDF). Det kgl. Danske Videnskabernes Selskabs Forhandlinger. i: i–24.
- ^ Boysen JP (1913). "Über die Leitung des phototropischen Reizes in der Avena-koleoptile". Berichte der Deutschen Botanischen Gesellschaft. 31: 559–566. doi:x.1111/j.1438-8677.1913.tb06990.10.
- ^ Larsen P (1960). "Peter Boysen Jensen 1883-1959". Constitute Physiology. 35: 986–988. doi:10.1104/pp.35.vi.986. }
- ^ a b Croker Due south, MacMillan J, Gaskin P. "Nature of auxin". Mendipweb.
- ^ a b Leyser O (January 2018). "Auxin Signaling". Plant Physiology. 176 (1): 465–479. doi:10.1104/pp.17.00765. PMC5761761. PMID 28818861.
- ^ a b c
- ^ Fendrych Chiliad, Akhmanova M, Merrin J, Glanc M, Hagihara S, Takahashi K, et al. (July 2018). "Rapid and reversible root growth inhibition by TIR1 auxin signalling". Nature Plants. four (seven): 453–459. doi:10.1038/s41477-018-0190-ane. PMC6104345. PMID 29942048.
- ^ Mashiguchi Yard, Tanaka M, Sakai T, Sugawara S, Kawaide H, Natsume M, et al. (November 2011). "The main auxin biosynthesis pathway in Arabidopsis". Proceedings of the National Academy of Sciences of the Usa. 108 (45): 18512–18517. Bibcode:2011PNAS..10818512M. doi:ten.1073/pnas.1108434108. PMC3215075. PMID 22025724.
- ^ Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, et al. (May 2006). "Pin proteins perform a rate-limiting role in cellular auxin efflux". Scientific discipline. 312 (5775): 914–918. Bibcode:2006Sci...312..914P. doi:10.1126/scientific discipline.1123542. PMID 16601150. S2CID 28800759.
- ^ Swarup R, Péret B (2012-01-01). "AUX/LAX family unit of auxin influx carriers-an overview". Frontiers in Found Science. 3: 225. doi:10.3389/fpls.2012.00225. PMC3475149. PMID 23087694.
- ^ Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, et al. (November 1999). "An auxin-dependent distal organizer of design and polarity in the Arabidopsis root". Cell. 99 (5): 463–472. doi:10.1016/S0092-8674(00)81535-4. hdl:1874/21099. PMID 10589675. S2CID 8041065.
- ^ Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (November 2005). "Patterns of auxin transport and cistron expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem". Current Biological science. 15 (21): 1899–1911. doi:10.1016/j.cub.2005.09.052. PMID 16271866. S2CID 14160494.
- ^ Tan S, Zhang X, Kong W, Yang Xl, Molnár G, Vondráková Z, et al. (May 2020). "The lipid code-dependent phosphoswitch PDK1-D6PK activates PIN-mediated auxin efflux in Arabidopsis". Nature Plants. 6 (5): 556–569. doi:10.1038/s41477-020-0648-9. PMID 32393881. S2CID 218593545.
- ^ a b Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, et al. (May 2009). "A regulated auxin minimum is required for seed dispersal in Arabidopsis". Nature. 459 (7246): 583–586. Bibcode:2009Natur.459..583S. doi:x.1038/nature07875. PMID 19478783. S2CID 4411776.
- ^ Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazímalová E (Dec 29, 2009). "The Pin-FORMED (Pivot) protein family unit of auxin transporters". Genome Biology. 10 (12): 249. doi:10.1186/gb-2009-10-12-249. PMC2812941. PMID 20053306.
- ^ Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (Nov 2005). "Patterns of auxin send and gene expression during primordium evolution revealed by live imaging of the Arabidopsis inflorescence meristem". Current Biological science. 15 (21): 1899–1911. doi:10.1016/j.cub.2005.09.052. PMID 16271866. S2CID 14160494.
- ^ "Arabidopsis CUP-SHAPED COTYLEDON3 Regulates Postembryonic Shoot Meristem and Organ Boundary Formation". Wikigenes. 2006.
- ^ Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM (Nov 2005). "Patterns of auxin transport and gene expression during primordium evolution revealed by live imaging of the Arabidopsis inflorescence meristem". Current Biology. xv (21): 1899–1911. doi:10.1016/j.cub.2005.09.052. PMID 16271866. S2CID 14160494.
- ^ Aloni R, Aloni Due east, Langhans 1000, Ullrich CI (May 2006). "Office of cytokinin and auxin in shaping root compages: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism". Annals of Botany. 97 (5): 883–893. doi:10.1093/aob/mcl027. PMC2803412. PMID 16473866.
- ^ Chambers (1999). Scientific discipline and Technology Lexicon . ISBN978-0-550-14110-1.
- ^ "That is why plants grow towards the light!". Jiří Friml Lab. VIB (the Flanders Institute for Biotechnology. 2012. Archived from the original on 2018-12-15.
- ^ Nemhauser JL, Feldman LJ, Zambryski PC (September 2000). "Auxin and ETTIN in Arabidopsis gynoecium morphogenesis". Development. 127 (xviii): 3877–3888. doi:ten.1242/dev.127.18.3877. PMID 10952886.
- ^ Friedman We (June 2009). "Evolution. Auxin at the evo-devo intersection". Science. American Clan for the Advancement of Science. 324 (5935): 1652–1653. Bibcode:2009Sci...324.1652F. doi:ten.1126/science.1176526. PMID 19556491. S2CID 206521265.
- ^ McSteen P, Malcomber S, Skirpan A, Lunde C, Wu X, Kellogg E, Hake S (June 2007). "barren inflorescence2 Encodes a co-ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize". Plant Physiology. 144 (2): 1000–1011. doi:10.1104/pp.107.098558. PMC1914211. PMID 17449648.
- ^ Yu YB, Yang SF (December 1979). "Auxin-induced Ethylene Product and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion". Institute Physiology. 64 (6): 1074–1077. doi:10.1104/pp.64.6.1074. PMC543194. PMID 16661095.
- ^ McSteen P (March 2010). "Auxin and monocot development". Cold Leap Harbor Perspectives in Biological science. 2 (3): a001479. doi:10.1101/cshperspect.a001479. PMC2829952. PMID 20300208.
- ^ The Manufacture Chore Strength II on 2,4-D Research Data
- ^ Mesejo C, Rosito S, Reig C, Martínez-Fuentes A, Agustí Yard (2012). "Synthetic Auxin 3,5,6-TPA Provokes Citrus clementina (Hort. ex Tan) Fruitlet Abscission past Reducing Photosynthate Availability". Journal of Plant Growth Regulation. 31 (2): 186–194. doi:10.1007/s00344-011-9230-z. S2CID 8338429.
- ^ Grossmann G (September 2007). "Auxin herbicide activity: lifting the veil step by pace". Plant Signaling & Behavior. two (5): 421–423. doi:10.4161/psb.two.5.4417. PMC2634233. PMID 19704620.
Farther reading [edit]
- Locascio A, Roig-Villanova I, Bernardi J, Varotto Southward (2014-08-25). "Electric current perspectives on the hormonal control of seed development in Arabidopsis and maize: a focus on auxin". Frontiers in Found Science. Frontiers. five: 412. doi:10.3389/fpls.2014.00412. PMC4142864. PMID 25202316.
johnswoperand1967.blogspot.com
Source: https://en.wikipedia.org/wiki/Auxin
Postar um comentário for "Complex Regulation of the Tir1/afb Family of Auxin Receptors"